If you’re a pharmaceutical company looking to introduce a new medicine to the Irish market, navigating the reimbursement process can be complex and time-consuming.
In this video you’ll gain a better understanding of the two routes to reimbursement in Ireland: Rapid Review and Health Technology Assessment (HTA).
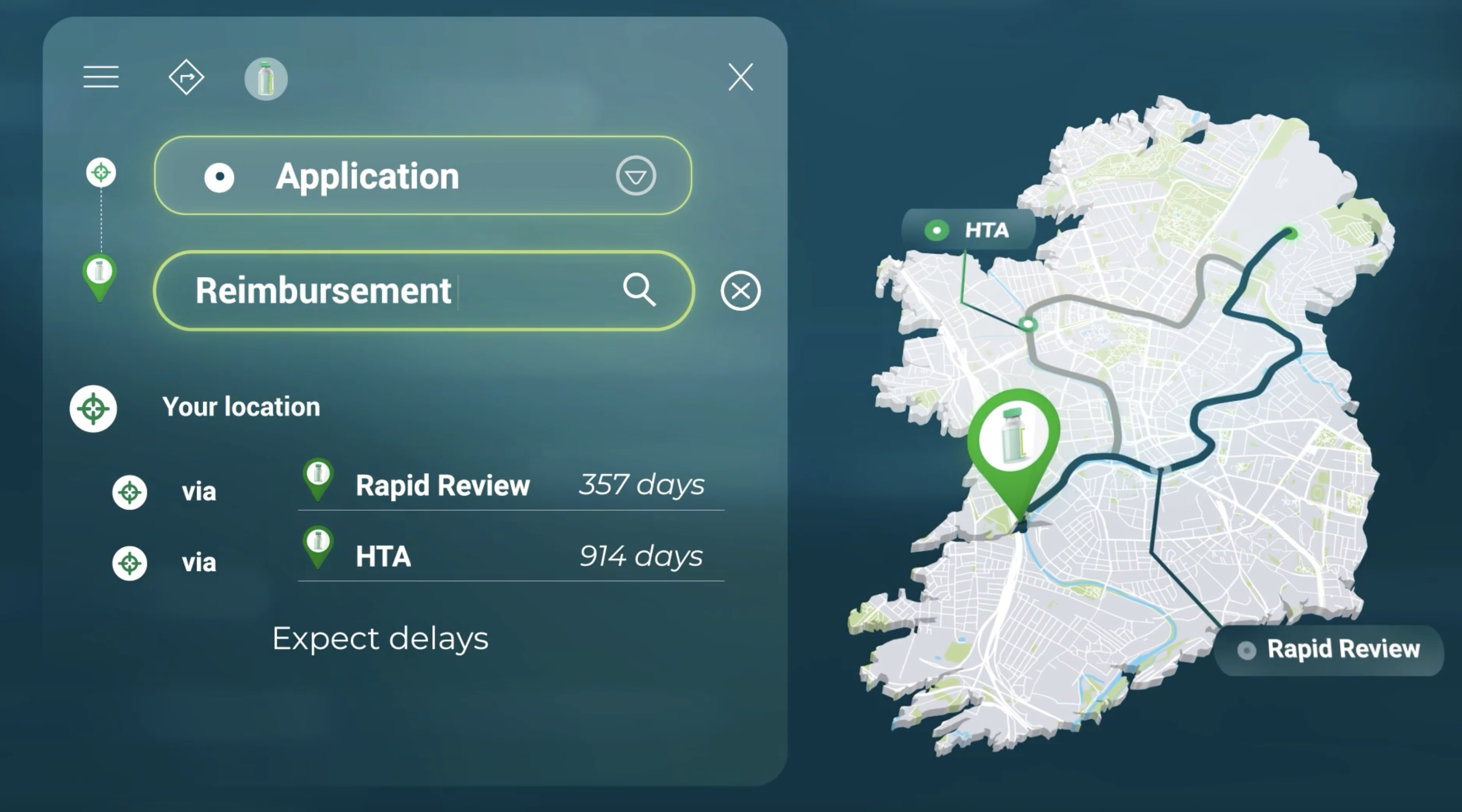
The reimbursement process is officially triggered by submitting an application to the Corporate Pharmaceutical Unit (CPU). This application includes a Rapid Review submission, which determines whether a HTA is required or not based on the relative clinical benefit and relative cost of the new medicine. If a HTA is required, it is assessed by the National Centre for Pharmacoeconomics (NCPE). Timelines to reimbursement vary between the two routes. For example, it takes 357 and 914 days for medicines to be reimbursed following a Rapid Review and HTA respectively. The long timelines are due to price negotiations that are required post submissions to secure reimbursement.
Salutem Insights offers a comprehensive solution to help pharmaceutical companies navigate the Irish reimbursement process with ease. Their services include HTAs, Rapid Reviews, systematic literature reviews, expert elicitation, and burden of illness studies. By partnering with Salutem Insights, you can ensure that your reimbursement application is handled efficiently and effectively, allowing you to focus on bringing your medicine to patients in need.
Don’t let the reimbursement process be a roadblock to introducing your medicine in Ireland. Contact Salutem Insights today to learn more about how they can help you succeed.